23,000+ patients using Philips InCourage vest therapy are living, breathing proof
A powerful pairing
Yearly rate of hospitalization decreased

62%
Ability to clear lungs
Good-excellent rating increased

63%

Respiratory health
Good-excellent rating increased

46%

Antibiotic use decreased

13%
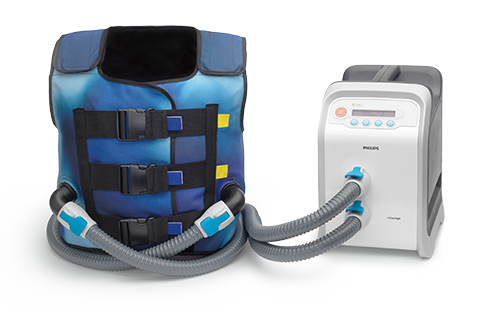
Why choose the Philips InCourage system for your patients?
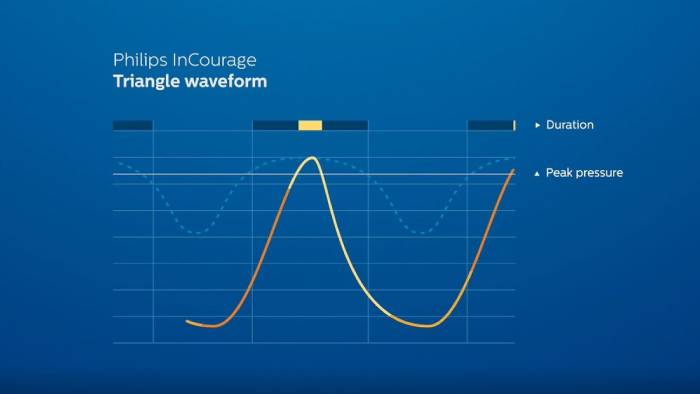
RespirTech’s services support the patient at every step of the journey to better breathing.- The InCourage system utilizes triangle waveform technology, which delivers a brief, intense, chest physical therapy (CPT)-like “thump” to the chest. Research has shown triangle waveform aids in clearing up to 20% more mucus than competing technology.3,4
The InCourage system’s active venting feature is designed to enable deep breaths during therapy, enhance comfort and help encourage adherence.
How to prescribe InCourage vest therapy for your patients:
A RespirTech sales representative in your territory would be happy to help answer any questions you may have about obtaining the Philips InCourage system for your patients.

Call 800.793.1261 or fax prescriptions to 800.962.1611

To request information by email, use our online inquiry form below.
Real people. Real results. Proof isn’t just numbers, it’s people who had trouble breathing from various chronic respiratory conditions, who shared how they are now breathing better and enjoying life with help from InCourage vest therapy.
“When my patients ask if their quality of life can improve, I tell them yes.”-Frederic D. Seifer, MD, Pulmonologist‡‡Dr. Eric Seifer is a paid consultant to RespirTech and a member of its advisory panel. Results from case studies are not predictive of results in other cases. Results may vary.
“It’s completely changed my life. The InCourage system has done what they say it should do. It cleaned my lungs, it’s kept them clean.”-Marjorie, bronchiectasis and COPD patient
“My shortness of breath has improved dramatically. Physically I just feel so much better.”-Dale, COPD patient
References
1. Methodology: Phone surveys at regular intervals with bronchiectasis patients using the InCourage system. Data collection began 10/01/2013. As of 05/31/2021, the total cohort was 23,213 patients; 21,049 patients completed the baseline survey; 13,303 patients in 1-month cohort; 9,569 in 6-month cohort; 7,720 in 12-month cohort 2. RespirTech's bronchiectasis patient outcomes program consists of follow-up calls at periodic intervals for up to two years to encourage HFCWO adherence and ensure the device is properly set for individual needs. 3. Milla CE, Hansen LG, Weber A, Warwick WJ. High-Frequency chest compression: effect of the third generation compression waveform. Biomed Instrum Technol 2004; 38:322-328. Note: 8 CF patient study comparing triangular vs. sine waveform technology. 4. Kempainen RR, Williams CB, Hazelwood A, Rubin BK, Milla CE. Comparison of high-frequency chest wall oscillation with differing waveforms for airway clearance in cystic fibrosis. Chest. 2007;132(4):1227-1232.